Lessons I Learned From Info About How To Find Out How Many Protons

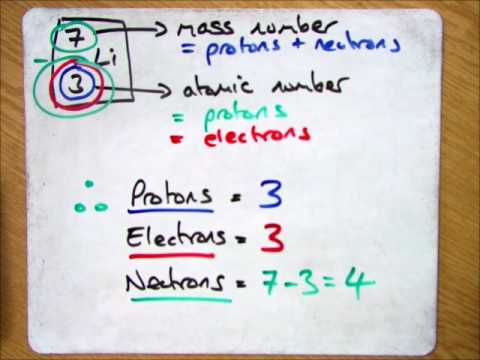
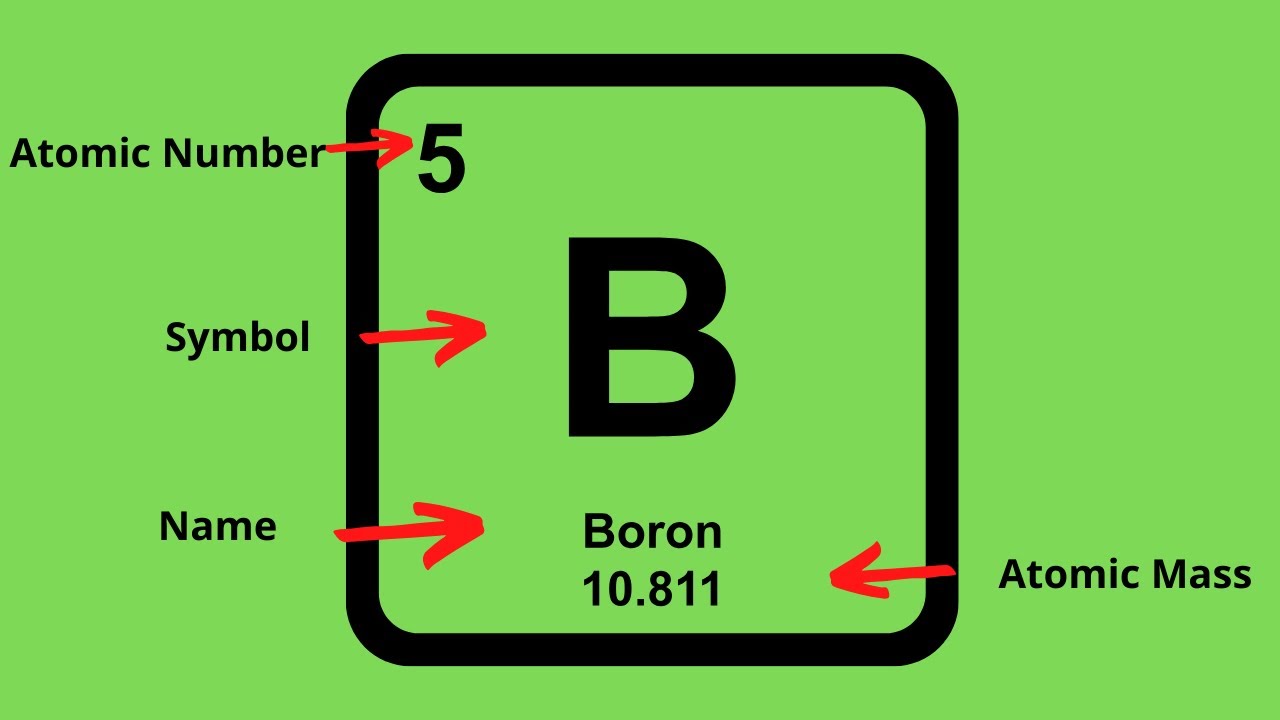
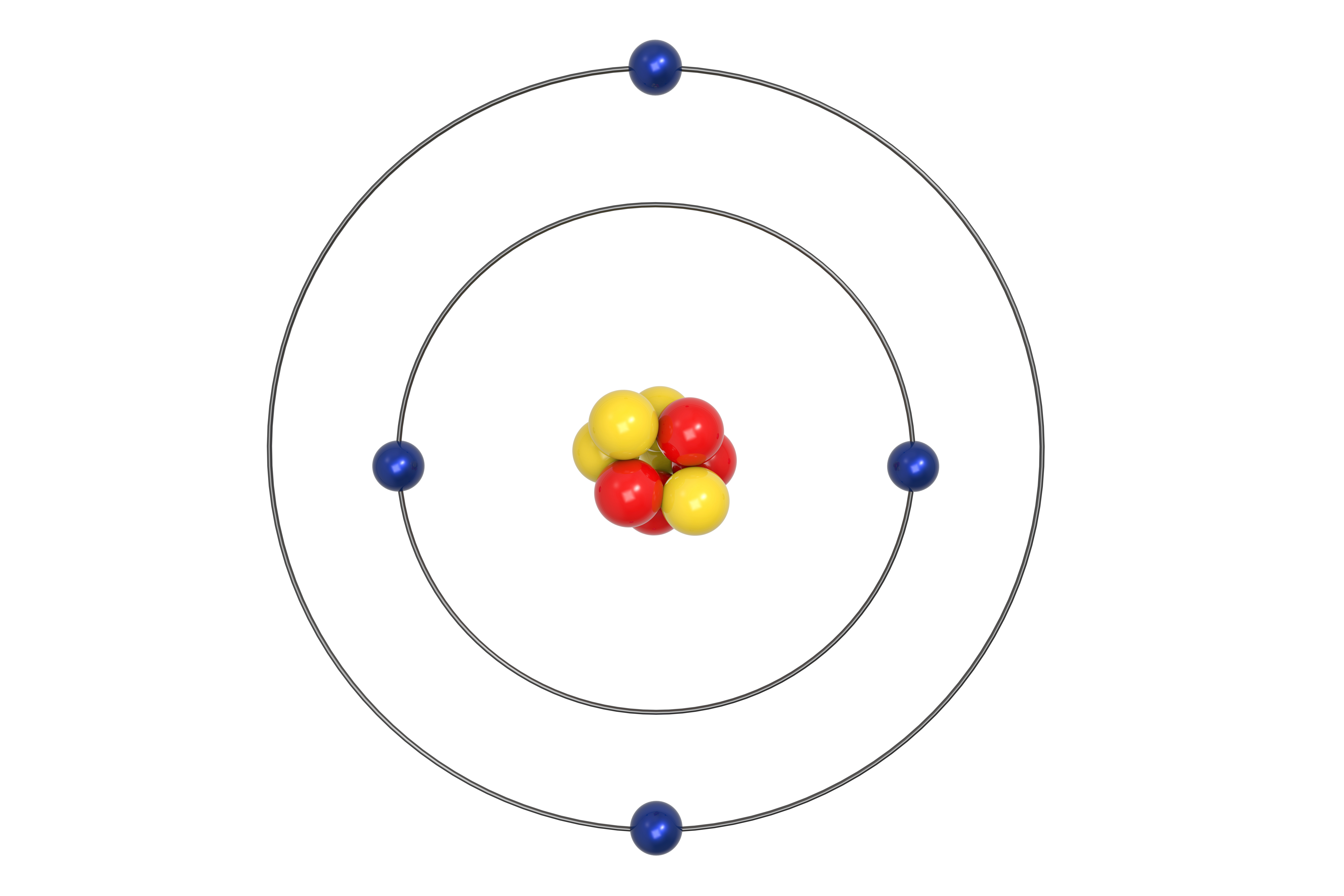
We know that an equal number of protons of atomic number are located in the nucleus of the element and electrons equal to protons are in orbit outside the nucleus.
How to find out how many protons. Of protons 3 of neutrons = mass no. Rules to finding number of protons, neutrons, and electrons. Some simple fundamentals that students must be aware of are as follows:
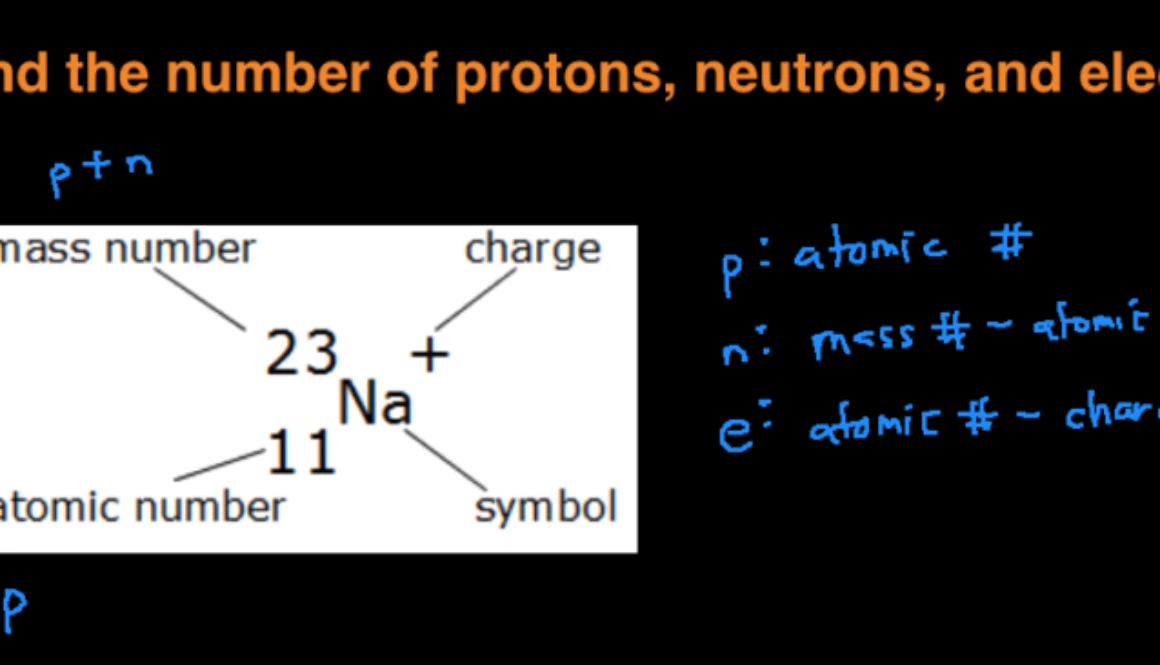
Of element 2 of electrons = no. How to calculate the number of protons, electrons and electrons in an atom? Number of protons = atomic number number of electrons = number of protons = atomic number number of.
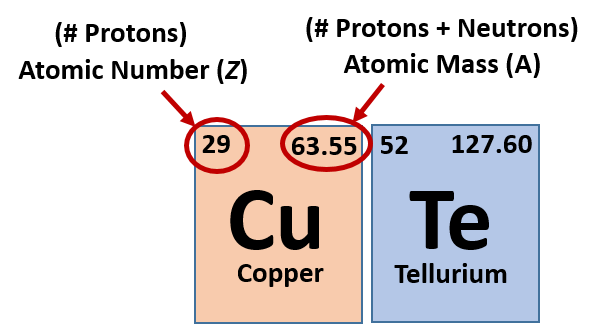
Therefore, the total number of protons and. Therefore, the total number of protons and. Atomic mass (a) = nucleus mass = total mass of protons and neutrons (p + n) again, the mass of each proton and neutron is about 1amu.
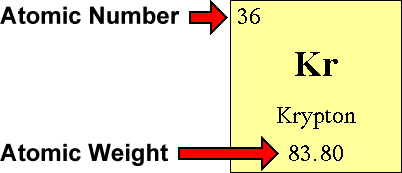
The atomic quantity will say you how many protons produce up a only. # of protons = atomic number. Atomic mass (a) = nucleus mass = total mass of protons and neutrons (p + n) again, the mass of each proton and neutron is about 1amu.
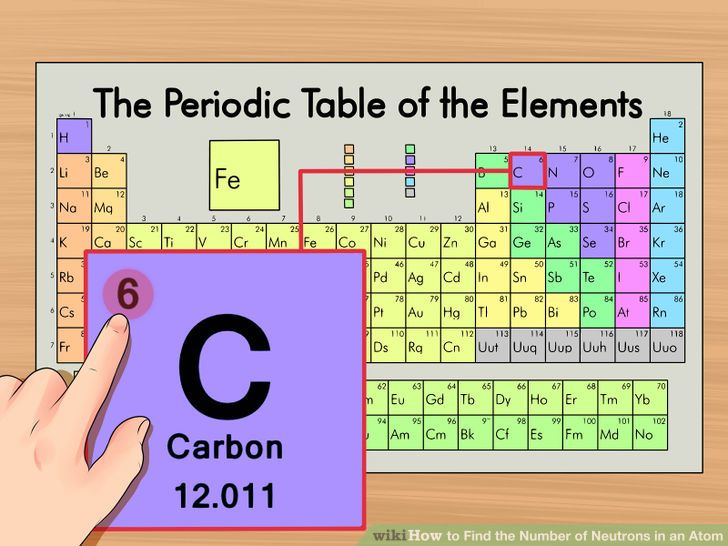
We know that an equal number of protons of atomic number are located in the nucleus of the element and electrons equal to protons are in orbit outside the nucleus. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for the element carbon (c). How do you find electrons in an atom?
Atomic mass (a) = nucleus mass = total mass of protons and neutrons (p + n) again, the mass of each proton and neutron is about 1amu. Atomic mass (a) = nucleus mass = total mass of protons and neutrons (p + n) again, the mass of each proton and neutron is about 1amu. 1 of protons = atomic no.







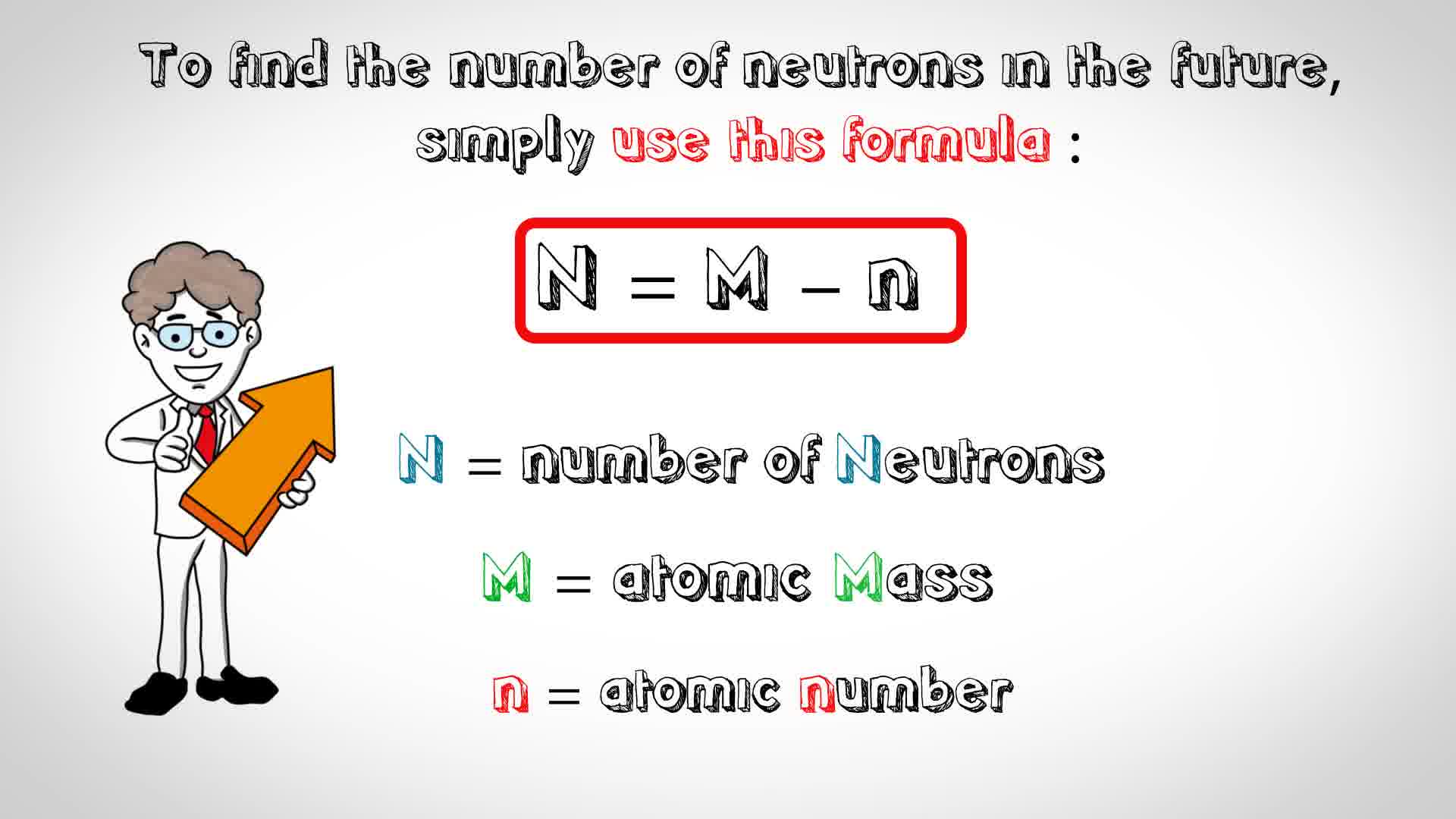
/GettyImages-523446050-5897be0a5f9b5874ee7c9fa6.jpg)
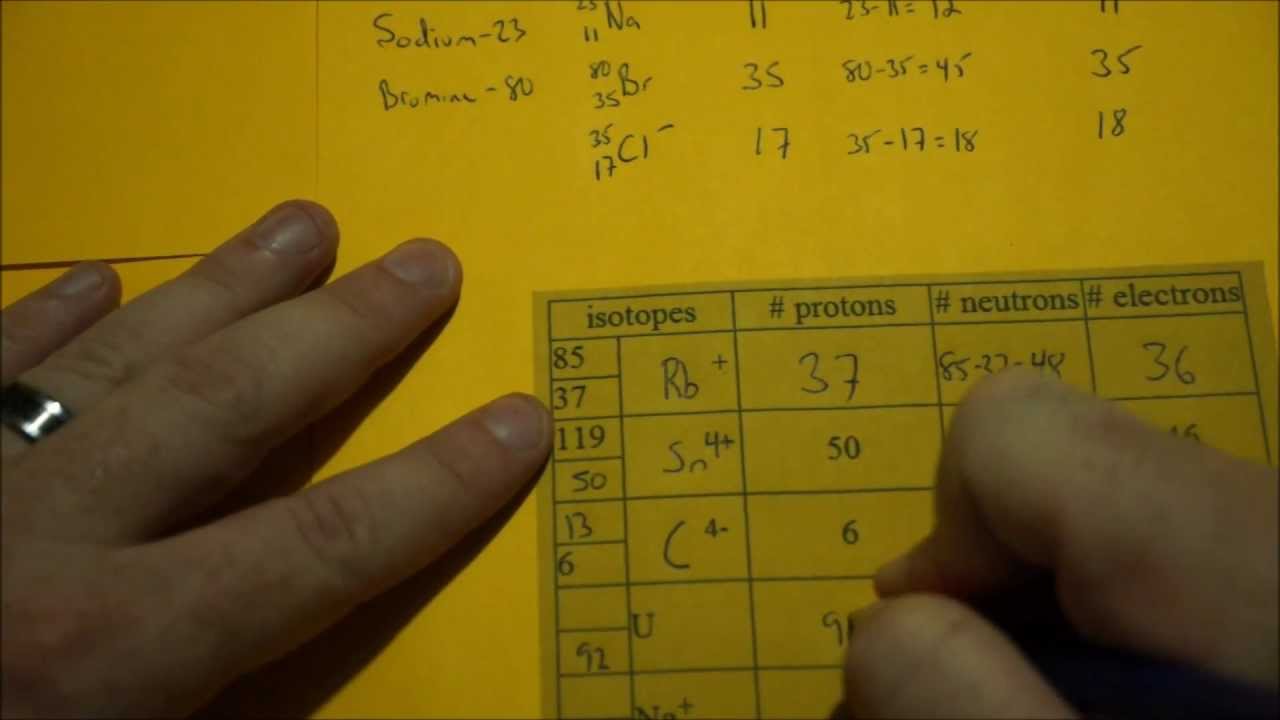

![Ap Chemistry: Periodic Table] How Many Protons And Neutrons Are In The Nucleus Of Each Of The Following Atoms? In A Neutral Atom Of Each Element, How Many Electrons Are Present? :](https://preview.redd.it/r6vqp1tajue31.jpg?width=640&crop=smart&auto=webp&s=be986430718a1b7ce1459862a328513c97c854fa)